TOKYO, April 27, 2021 – FUJIFILM Corporation (President: Kenji Sukeno) is pleased to announce that 23 of its products in various business fields including the instax series of instant cameras, the X series of digital cameras and endoscopic diagnosis support function CAD EYE™ have been awarded the “iF Design Award 2021”, sponsored by the iF International Forum Design (Hannover, Germany).
Fujifilm sets its highest record with 23 products winning the prestigious award in recognition of not only the products’ beautiful appearance but also their design that achieves advanced performance and excellent operability.
The iF Design Award is a prestigious international design award launched in 1953, and is considered one of the top three global design awards, alongside “Red Dot Design Award” in Germany and “the International Design Excellence Awards (IDEA)” in the United States. This year, there were 9,509 entries from 52 countries and regions around the world.
In developing all of its products and services, Fujifilm not only pursues functionality and performance, but also strives to develop designs that bring out the best of the advanced functionality and performance. Fujifilm will continue to work on creating design that has beautiful appearance and reflects commitment to offering operability for ease of use and comfort, thereby creating new product values.
With attention firmly on design excellence as one of the product values, Fujifilm will continue to develop superior products, encouraged by the honor of having many products chosen for the iF Design Award.
![[logo]iF Design Award 2021](https://asset.fujifilm.com/www/jp/files/2021-04/66daf13058e89d7ac769c1e2ca5a3f83/news_6493_01.jpg)
“iF Design Award 2021” winners (23 products)
①Mirrorless digital camera “FUJIFILM X-T4”

②Mirrorless digital camera “FUJIFILM X-S10”

③Mirrorless digital camera “FUJIFILM X-T200”

④Digital camera function “Film Simulation”

⑤Interchangeable lens for X series digital cameras
“FUJINON Lens XC35mmF2”

⑥Interchangeable lens for X series digital cameras
“FUJINON Lens XF8-16mmF2.8 R LM WR”

⑦Interchangeable lens for X series digital cameras
“FUJINON Lens XF50mmF1.0 R WR”

⑧Interchangeable lens for GFX series digital cameras
“FUJINON Lens GF30mmF3.5 R WR”

⑨Interchangeable lens for GFX series digital cameras
“FUJINON Lens GF100-200mmF5.6 R LM OIS WR”

⑩Ultra-short throw projector
“FUJIFILM PROJECTOR Z8000”

⑬8K-compatible broadcast zoom lens
“FUJINON HP66×15.2-ESM”

⑭8K-compatible broadcast zoom lens
“FUJINON HP12×7.6ERD-S9”

⑮Focus position demand unit
“FUJINON EPD-51A-D02/F02”
(Accessory for broadcast lenses)

⑯Zoom lens for cinema cameras
“FUJINON Premista19-45mmT2.9”

⑰Binoculars
“FUJINON HYPER-CLARITY HC8×42/10×42”

⑱Smartphone app for “instax mini Link”

⑲Instant camera “instax SQUARE SQ1”

⑳Automated clinical chemistry analyzer
“DRI-CHEM NX600”

㉑Endoscopic diagnosis support function
“CAD EYE™”
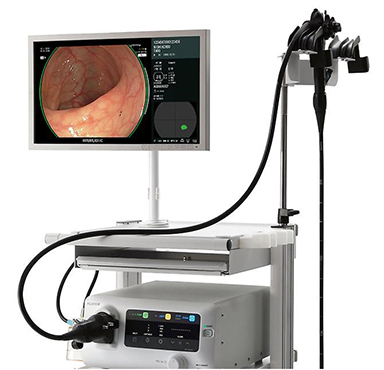
㉒“Expansion Unit EX-1” for endoscopy

㉓Compact digital X-ray cart system
“FUJIFILM DR CALNEO AQRO”
(Product name in Europe: "FDR nano")

Contact
Media Contact
FUJIFILM Holdings Corporation
Corporate Communications Division
Public Relations Group
* Please note that contents in this website are current as of the date of the press announcement and may be subject to change without prior notice. Information in each release, including the product availability, specification, prices, contacts, are those at the time of publication.
* This news release is issued by FUJIFILM Corporation in Japan.
* Fujifilm makes no representation that products on this news release are commercially available in all countries and regions(including US and EU). Approved uses of healthcare products vary by country and region.








