Nong Khai, 16 December 2025 — FUJIFILM (Thailand) Ltd. conducted a liver cancer screening initiative for over 100 high-risk individuals at Nong Khai Hospital in the Northeast region to support early detection. The screening program marks Thailand’s first use of the GALAD Score. The GALAD Score is an advanced diagnostic system that combines three blood-based HCC biomarkers - AFP, PIVKA-II, and AFP-L3 – together with patient demographic data (gender and age) to improve the accuracy of hepatocellular carcinoma (HCC) detection. In collaboration with Thailand Hepatitis Alliance and Nong Khai Hospital’s medical team, the initiative aims to raise awareness of the importance of early-stage liver cancer screening while introducing this innovative approach. It significantly enhances the detection efficacy of early-stage liver cancer compared to the traditional screening method that rely on AFP alone. By enabling more precise early diagnosis, this method increases the opportunity for timely liver cancer treatment.


Liver cancer accounts for over 14.4% of all cancers in Thailand, making it the most commonly diagnosed cancer and the leading cause of cancer-related deaths, with nearly 30,000 new cases annually and continuously rising incidence, particularly in the Northeast region where rates are highest. Liver cancer comprises two types: hepatocellular carcinoma (HCC), with major causes including hepatitis B and C virus infections, alcohol consumption, and fatty liver disease resulting from unhealthy dietary habits and lack of physical activity; and cholangiocarcinoma, often linked to consuming raw freshwater fish, which poses risks of liver fluke infection. Liver cancer is considered a silent threat to society, as it typically presents no symptoms in early stages. Most patients seek medical attention only after the disease has progressed, significantly reducing treatment success rates. Early detection of liver cancer is therefore critical to treatment outcomes, as patients diagnosed in early stages can access effective treatments such as surgery, ablation, or liver transplantation, often with successful recoveries.

Prof. Dr. Wattana Sukeepaisarnjaroen, Gastrointestinal unit, Department of Medicine, Srinagarind Hospital,Faculty of Medicine, Khon Kaen University and President of Thailand Hepatitis Alliance, said, “Current research on new biomarkers enhances liver cancer screening efficacy. Using three blood-based HCC biomarkers with GALAD Score alongside ultrasound enables accurate early-stage screening compared to the traditional methods such as single-biomarker approach. We urge government agencies to promote nationwide screening and support adoption of advanced biomarker technologies to increase access to effective screening services throughout the country.”


In this screening initiative, the Thailand Hepatitis Alliance has joined as a partner. The organization's mission focuses on education, prevention, and reducing hepatitis incidence in Northeast Thailand. Through collaboration with healthcare institutions, the Alliance works to raise awareness about viral hepatitis risks and promote screening among high-risk populations, ultimately aiming to reduce the burden of liver disease in the region.
Dr. Rudeemon Sakulkoo, Director of Nong Khai Hospital, said, “Nong Khai Hospital's vision is to serve as the Mekong Subregion health hub. Our study with the Thailand Hepatitis Alliance found that 12% of residents carry hepatitis B or C. This initiative screens patients using the GALAD Score, a simple, rapid, and accurate approach that enhances early-stage detection capability. This enables prompt treatment and potential recovery, aligned with our commitment to reducing mortality and elevating patients' quality of life."
Dr. Jamrus Pongpit, Gastroenterologist and Hepatologist at Nong Khai Hospital, added, “The Northeast has the highest hepatocellular carcinoma incidence, increasing annually. Most cases present at late stages, challenging treatment success. High-risk groups requiring screening include hepatitis B and C carriers, cirrhosis patients, alcohol drinkers, and those with fatty liver disease. The most effective measures to reduce patient numbers and mortality rates involve risk reduction and increased screening campaigns for hepatitis viruses to facilitate treatment access. Current research demonstrates that using three blood-based HCC biomarkers—AFP, AFP-L3, and PIVKA-II—with GALAD Score calculation can enable more accurate early-stage diagnosis of hepatocellular carcinoma.”
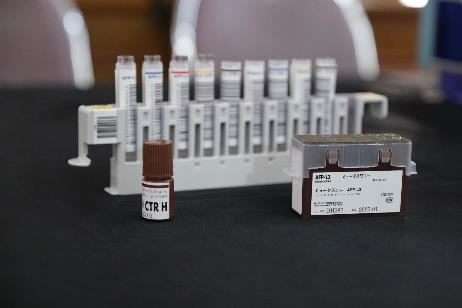

Highlights of the technology deployed in this screening initiative at Nong Khai Hospital include the HCC three-marker panel: AFP, PIVKA-II, and AFP-L3. AFP-L3, a blood-based liver cancer biomarker developed in Japan, enables detection of HCC that may not be detected by ultrasound in the early stages of tumor development, supporting rapid treatment. This technology has contributed to Japan achieving a higher detection rate of early-stage HCC (over 66%*). Beyond Japan-made AFP-L3, Fujifilm also employs the 'μTASWako i30', a fully automated immunoanalyzer specifically tailored for HCC diagnosis. This contributes to reducing preventable deaths by enhancing early-stage HCC detection.
Mr. So Maruo, Managing Director of FUJIFILM (Thailand) Ltd., stated, "Fujifilm continues to drive comprehensive medical solutions to support the healthcare of Thai people across multiple regions. Introducing the GALAD Score, which integrates three HCC biomarkers in this screening initiative, significantly enhances the accuracy of early-stage liver cancer detection compared to existing approaches. This enables patients to receive timely treatment and increases the likelihood of successful outcomes. Today's pilot program in Nong Khai Province represents a significant milestone for us. Moving forward, we remain committed to raising awareness among high-risk groups about the importance of regular screening, while supporting the adoption of effective liver cancer screening approaches. We believe that access to accurate diagnostic innovations leading to timely treatment will help reduce preventable loss of life and enhance well-being for Thai people, aligned with Fujifilm Group’s purpose of 'Giving our world more smiles.'"
Reference:
* Presentation by Prof. Masatoshi Kudo at Kindai University at METI-THASL project seminar in December, 2020










